This phenomenon can seem a bit counterintuitive at first because most materials contract when they transition from liquid to solid. However, water behaves differently due to the unique properties of its molecules and the hydrogen bonds between them.
(I)The Molecular Structure of Water
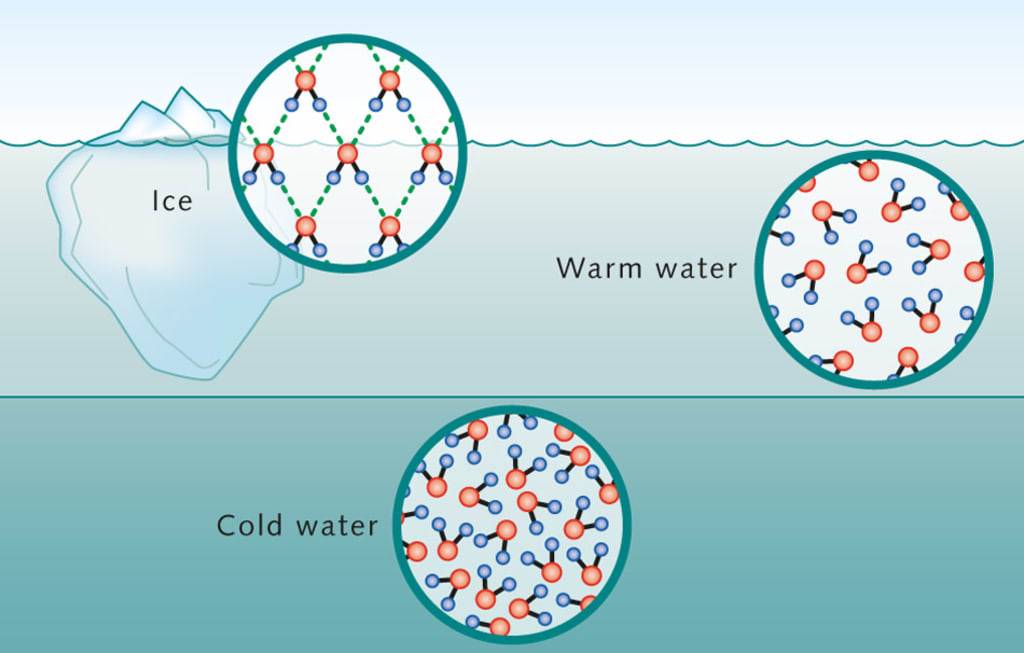
At the heart of this unusual behavior is the molecular structure of water. A water molecule consists of two hydrogen atoms covalently bonded to one oxygen atom, forming a V-shape. This structure gives water its polarity, meaning the oxygen atom has a slight negative charge, and the hydrogen atoms have a slight positive charge. This polarity allows water molecules to form hydrogen bonds, a type of weak but significant attraction between the positively charged hydrogen atoms of one molecule and the negatively charged oxygen atoms of another.
In its liquid state, water molecules are constantly moving, and the hydrogen bonds are continually forming and breaking as the molecules slide past one another. This fluidity allows water molecules to pack relatively closely together, giving the liquid its characteristic density.
(II)The Role of Hydrogen Bonds in Freezing
When water starts to freeze, the molecules slow down because the temperature is decreasing. As the water reaches 0°C (32°F), the hydrogen bonds begin to stabilize, and the water molecules start to arrange themselves in a regular pattern. This pattern, known as a crystalline lattice, takes up more space than the liquid form. The specific arrangement of the water molecules in this lattice structure is key to understanding why water expands upon freezing.
In the solid state (ice), each water molecule is hydrogen-bonded to four neighboring water molecules, forming a tetrahedral structure. These bonds hold the molecules apart, creating open spaces between them. This arrangement is not as tightly packed as in the liquid state, which means that ice has a lower density than liquid water. As a result, ice expands and becomes less dense than water, which is why it floats.
(III)Why Does Water's Expansion Matter?
Water's expansion upon freezing has several important implications. One of the most notable effects is the role it plays in the environment, particularly in bodies of water. Since ice floats on top of water, it forms an insulating layer that helps to regulate temperatures below the surface. This insulating effect is crucial for aquatic life, as it prevents the entire body of water from freezing solid, allowing organisms to survive even in cold conditions.
Additionally, the fact that water expands when it freezes has significant consequences for the physical world. The expansion of water upon freezing is powerful enough to cause physical damage. For example, water that seeps into cracks in rocks or concrete can freeze, expand, and cause the material to crack or break apart. This process, known as frost wedging, is a key mechanism of weathering in nature. Over time, it can lead to the breakdown of mountains, cliffs, and other geological features.
(IV)The Anomaly of Water’s Expansion
Water's expansion upon freezing is an anomaly in the physical world because most substances behave in the opposite manner. When a material freezes, the molecules typically slow down and settle into a more ordered structure, which causes them to pack more tightly together. This leads to a decrease in volume and an increase in density. However, water defies this pattern due to the nature of the hydrogen bonds.
The explanation for water's behavior can be traced back to the concept of "structural anomalies." These anomalies arise when the arrangement of atoms or molecules in a substance leads to behavior that deviates from the norm. In the case of water, its hydrogen bonds cause a unique arrangement in the solid state that requires more space.
(V)Why is this Important for Life?
Water’s ability to expand upon freezing is vital for life on Earth. For one, it plays a role in Earth's climate and weather systems. Without this property, bodies of water would freeze from the bottom up, making it difficult for marine life to survive. Ice on the surface would sink, and the entire water body would eventually become frozen solid. The expansion of ice ensures that it remains on the surface, providing a protective layer and allowing organisms to thrive in the liquid water beneath.
Furthermore, the expansion of water when it freezes has had an impact on the development of Earth's landscape. The weathering caused by ice expansion has shaped the surface of the planet for millions of years, creating unique geological formations.
Conclusion
The expansion of water when it freezes is an extraordinary property that is a result of its molecular structure and the hydrogen bonds that hold its molecules together. This behavior is essential to life on Earth, affecting everything from the environment to the survival of marine life and the physical landscape. It serves as a reminder of how small, seemingly insignificant properties of substances can have far-reaching impacts on the world around us.